Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Woours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Woours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Conductive coatings, as functional coatings, can be used to create coatings that conduct current and remove accumulated charges. They have wide applications in antistatic, electromagnetic shielding, corrosion protection, new energy, and electronic information technology.
Conductive fillers, as core functional components in conductive coatings, serve to create charge transfer pathways within the coating. They also influence the coating’s application performance and adjust its gloss.
With technological innovation and advancement, the development of conductive fillers has undergone numerous iterations.
1.Conductive mechanism of conductive fillers
It is generally believed that the conductivity of a coating formed after mixing conductive fillers with a coating base is the result of a competition between the conductive channel theory, the tunneling effect, and the field emission effect.
The conductive channel theory states that when the volume fraction of the conductive filler in a coating reaches a certain value, the conductive particles in the coating contact each other, forming chain-like conductive channels, thus making the coating conductive. The tunneling effect states that during the mixing process, the resin sometimes encapsulates the filler, increasing the distance between the fillers. When the conductive filler content in the coating is relatively low, thermal vibrations under the influence of an applied electric field can cause carriers to jump directly between the filler particles, forming a conductive channel. The field emission effect is a special type of tunneling effect, in which high voltage excites carriers to jump through the resin layer to another filler particle.
In addition, other theories explain the conductive mechanism of coatings, such as thermodynamics and effective medium theory. However, due to their limited applicability or loopholes, these theories have not been widely accepted.

2.Conductive fillers made of metals and their oxides
When used as conductive fillers, metals can effectively transmit and disperse current and exhibit excellent electrical conductivity. However, they are relatively expensive and prone to precipitation in the base material, resulting in uneven distribution of components in the coating, which in turn affects overall conductivity. Furthermore, metals are susceptible to oxidation, which can reduce or even completely eliminate the coating’s conductivity. These drawbacks limit their application as fillers. Commonly used metal fillers include silver, aluminum, nickel, zinc, and copper.
Silver is one of the most conductive metals, but its high cost limits its use to specialized applications requiring high electromagnetic shielding effectiveness. Aluminum has lower conductivity than silver, but is lightweight and inexpensive. However, it is chemically active and easily corroded, so in water-based coating systems, aluminum powder must be surface-modified. Nickel’s poorer conductivity than silver and aluminum and its carcinogenicity limit its application, but its good corrosion resistance and low cost make it suitable for architectural applications.
Metal oxides exhibit semiconductor conductivity and, compared to metal fillers, are less expensive, have lighter colors, are more corrosion-resistant, and offer superior decorative properties. Common ones include titanium dioxide, zinc oxide, tin oxide, cobalt oxide, etc.

3.Carbon-based conductive fillers
Carbon-based materials have evolved from natural graphite and carbon black to carbon fibers, carbon nanotubes (CNTs), and graphene. Due to their advantages such as light weight, excellent conductivity, non-toxicity, and good adhesion, they are widely used as conductive fillers in coatings.
Carbon black has excellent light and chemical stability, but its numerous micropores on its surface result in a large surface area and are prone to agglomeration when dispersed in aqueous systems. Therefore, a dispersant is required for its use as a filler. Carbon fibers are high-strength, high-modulus fibers with a carbon content of over 90%. They are often used as reinforcements in composites with resins and metals. In the coatings industry, their light weight and excellent electrical and thermal conductivity make them suitable for the preparation of conductive, heat-generating, or thermally conductive coatings.
CNTs are one-dimensional conductive materials with a unique structure. Due to strong van der Waals forces between the nanotubes, they are prone to agglomeration. Furthermore, their chemical inertness makes them less reactive to components such as the binder and solvent. Therefore, the use of CNTs as coating fillers requires a solution to the dispersion problem in the system. One solution is mechanical dispersion, such as ultrasonic treatment, mechanical grinding, and high-speed stirring. Another is functionalization, including covalent and non-covalent functionalization. Furthermore, the addition of dispersants can improve the dispersion of CNTs in the matrix.
Graphene, a two-dimensional layered nanostructured conductive material, is impermeable to corrosive media such as oxygen and water in air, making it widely used in corrosion protection. Typically, van der Waals forces and stacking interactions between graphene layers impair its dispersion, making it prone to aggregation and deposition in solvents or polymers, which directly affects the coating’s conductive properties. Improving graphene dispersion is typically achieved by adding appropriate additives or performing functionalization. The resulting conductive coatings often also exhibit excellent mechanical and corrosion resistance.
4.Intrinsically Conductive Polymers (ICPs) Conductive Fillers
ICPs possess both intrinsic dopant conductivity and the ability to form films, making them a hot topic in the field of conductive coatings. Considering the comprehensive requirements of stability, conductivity, and water-based properties, polyaniline (PANi), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) are currently the most studied.
PANi exhibits excellent conductivity, thermal stability, and chemical stability. Its doping reaction is highly selective, allowing its electrical and chemical properties to be tuned by adding dopants. Covalent modification or composites with other materials can also address PANi’s performance deficiencies (such as corrosion resistance). Compared to PANi, PPy offers a simpler synthesis method, a safer, greener, and more cost-effective process. PEDOT:PSS is the most extensively studied and widely used commercial water-based dispersion in the field of ICPs. It overcomes the challenges of polythiophene, such as poor dispersibility, conductivity, and processability, and holds great promise for applications in flexible and wearable electronic devices.

5.Composite conductive fillers and new conductive fillers
Composite fillers typically consist of multiple conductive materials, which interact to form a conductive network, enhancing the filler’s conductivity. The differences in their physical and chemical properties improve the filler’s compatibility and dispersibility. Furthermore, the composite system is less susceptible to breakage or wear when subjected to external forces, improving the coating’s tensile strength and wear resistance. Currently, the most researched systems in this area are those combining ICPs with carbon materials or metal nanowires.
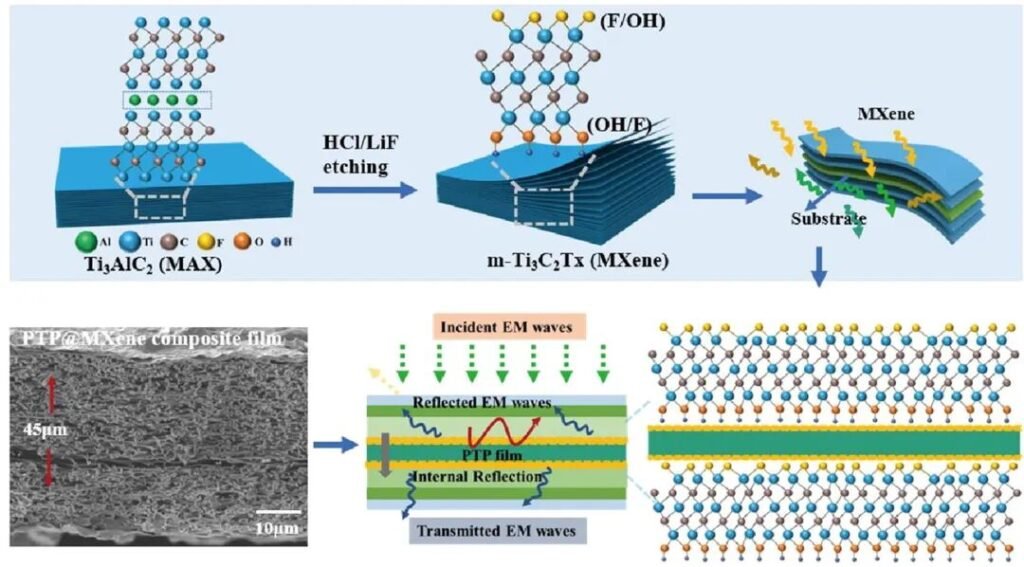
Among new fillers, new materials such as MXene and MOFs hold great potential in the field of conductive fillers and are a hot topic in research across various sectors, including energy, electronics, catalysis, sensing, and printing and dyeing. As two-dimensional, sheet-like nanomaterials with metallic and covalent bonds, MXene exhibits excellent conductivity, mechanical properties, surface activity, and high photothermal conversion efficiency. MOF is a crystalline framework material with intramolecular pores formed by self-assembly of metal ions or clusters and organic ligands through coordination bonds under certain conditions. It has the advantages of high porosity, size selectivity and structural diversity. It can be hybridized with metal elements, graphene, MXene and other materials to obtain better electrical and magnetic properties, thereby playing a unique role in the conductive coating sector of emerging industries.