Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Woours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Woours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM

Silanes are a class of organic/inorganic hybrids containing silicon groups. Their basic molecular formula is: R'(CH2)nSi(OR)3.
OR is a hydrolyzable group, and R’ is an organic functional group. Silanes contain two different chemical functional groups. One end can react with hydroxyl groups on the surface of inorganic materials (such as glass fiber, silicates, metals, and their oxides) to form a covalent bond; the other end can form a covalent bond with the resin, thereby combining two materials with very different properties and improving the performance of the composite material.
The silanization process can be described as a four-step reaction model:
In order to shorten the aging time required for on-site use of the treatment agent, the first step before using the silane treatment agent is to pre-hydrolyze it at a certain concentration.
① Hydrolysis reaction: During the hydrolysis process, condensation reactions between silanes are inevitable, forming oligosiloxanes. Too little oligosiloxane will prolong the on-site aging time of the silane treatment agent, affecting production efficiency. Too much oligosiloxane will cause the treatment agent to become turbid or even precipitate, reducing its stability and affecting treatment quality.
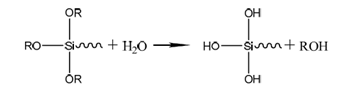
② Condensation reaction: Film-forming reaction is a key step affecting the quality of silanization. The quality of film-forming reaction directly affects the corrosion resistance of the coating and the adhesion of the paint film.
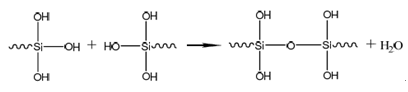
③ Film-forming reaction: Therefore, controlling parameters such as the pH value of the treatment agent is particularly important. Furthermore, higher requirements are placed on the workpiece surface condition before silanization:
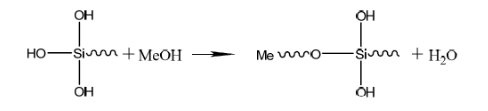
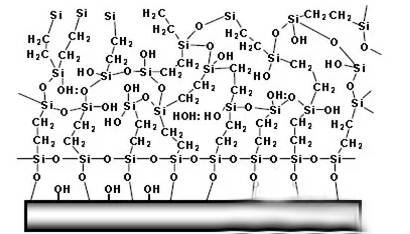
Where R is an alkyl substituent, and Me is a metal substrate. The metal silanization film layer after film formation is mainly composed of two parts: one is that on the metal surface, the silane treatment agent forms reaction ③ products through a film-forming reaction; the other is that a large amount of reaction ② products are formed through a condensation reaction, thereby forming a complete silane film. The microscopic model of the film-forming state on the metal surface can be described as the structure shown in picture.