Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Woours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM
Address
Room 2301C, 23rd Floor, Building 1, jinghu Commercial center, No, 34, Liangzhuang Street, Eri District, Zhengzhou City, Henan province
Woours
Monday to Friday: 7AM - 7PM
Weekend: 10AM - 5PM

Hydrophobicity and contact angle
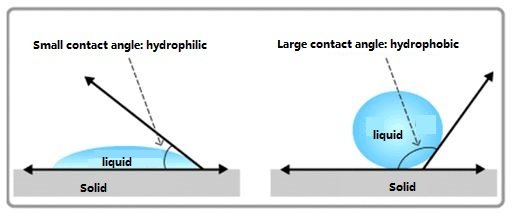
What is Contact Angle?
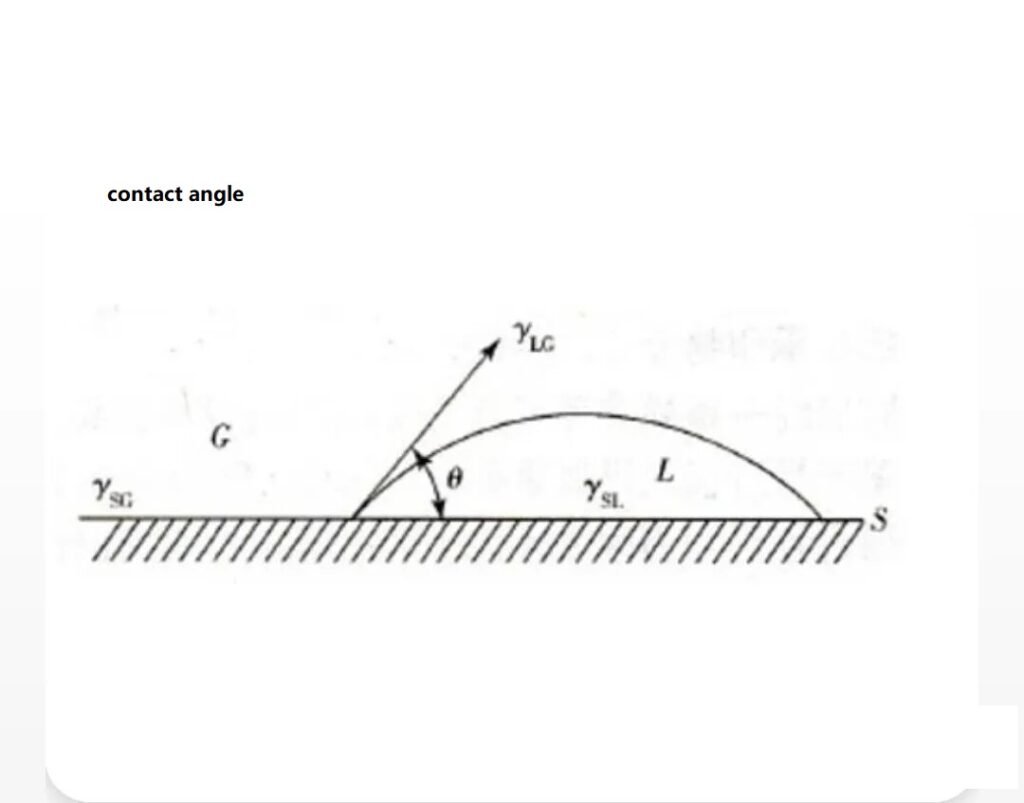
The contact angle is a measure of wettability.
It is the angle between the line tangent to the gas-liquid interface at the intersection of the gas, liquid, and solid phases, passing through the liquid, and the solid-liquid interface.
Application of contact angle
The contact angle of a liquid on a solid surface is an important parameter for measuring the wetting properties of a liquid on that surface.
Contact angle measurements can provide valuable information about solid-liquid and solid-gas interfacial interactions on a material’s surface.
Contact angle measurement technology is not only commonly used to characterize surface properties but also has important applications in the petroleum industry, flotation, pharmaceutical materials, chip industry, low-energy, non-toxic antifouling materials, inks, cosmetics, pesticides, printing and dyeing, papermaking, textile finishing, detergents, spray coatings, and wastewater treatment.
Contact angle test method
There are two common methods for measuring contact angle:
One is the topographic image analysis method
The other is the gravimetric method, the latter of which is often referred to as a wetting balance or penetrating contact angle meter. However, the topographic image analysis method is currently the most widely used and direct and accurate.
The topographic image analysis method works by placing a liquid droplet on the surface of a solid sample. A microscope lens and camera are used to capture an image of the droplet’s topography. Digital image processing and algorithms are then used to calculate the droplet’s contact angle from the image.
Calculation method
Contact angles are typically calculated based on a specific mathematical model, such as a liquid droplet, which can be considered as a segment of a sphere or cone. The contact angle is then measured using specific parameters such as width and height, or by direct fitting.
The Young-Laplace equation describes the relationship between the internal and external pressure differences at a closed interface and the curvature and interfacial tension of the interface. It can be used to accurately describe the external contour of an axisymmetric liquid droplet, thereby calculating its contact angle.
How to determine whether it is hydrophobic or hydrophilic?
If θ is less than 90°, the solid surface is hydrophilic, meaning liquids tend to wet the solid easily. The smaller the angle, the better the wettability.
If θ is greater than 90°, the solid surface is hydrophobic, meaning liquids do not wet the solid easily and tend to migrate across the surface. The wetting process is related to the interfacial tension of the system.
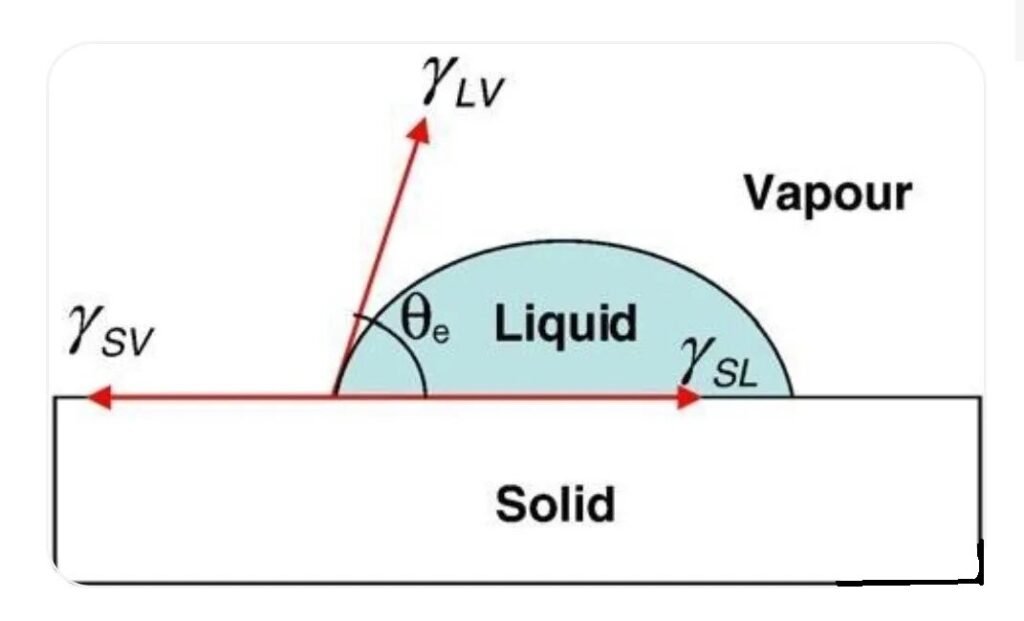
When a drop of liquid lands on a horizontal solid surface, at equilibrium, the resulting contact angle and interfacial tensions conform to the following Young’s equation:
γSV = γSL + γLV × cosθe
This equation can predict the following wetting conditions:
1) When θ = 0, complete wetting;
2) When θ < 90°, partial wetting or wetting;
3) When θ = 90°, it is the dividing line between wetting and non-wetting;
4) When θ > 90°, non-wetting;
5) When θ = 180°, complete non-wetting.